What is Metal Hydride (MH)
Alloy capable of absorbing and desorbing hydrogen
Hydrogen is the smallest atom. Hydrogen reacts with most elements to form hydrides or hydrogen compounds. In particular, it has long been known that metals react with hydrogen gas to produce metal hydrides. The rare earth metals (magnesium, palladium, titanium, zirconium, vanadium) readily react with hydrogen, absorbing a large amount of hydrogen between metal crystal lattices to form a metal compound. On the other hand, iron, nickel and the like are hard to react with hydrogen and react with hydrogen unless it is brought to high temperature and high pressure. In order to use a metal hydrogen compound as a hydrogen storage material, a hydrogen releasing ability is required. The rare earth metals have a high hydrogen storage capacity, but their release capacity is low. Therefore, Alloys were made with the rare earth metals and the metals which hardly react with hydrogen, which resulted in metals having both hydrogen absorbing capacity and desorbing capacity. This alloy is called Metal Hydride.(References: "New edition Metal Hydride - its properties and applications", Yasuaki Osumi, Agne Technology Center, 1993 (in Japanese))
Characteristics of Metal Hydride

Before pulverizing MH

MH powder LaNi5
・When pressurizing, hydrogen is absorbed, and when it is depressurized, hydrogen is desorbed.
・MH is pulverized into powder form to increase the reaction surface area.
・Average particle size is reduced as the number of hydriding cycles increases.
・MH expands with hydrogen absorption and contracts by hydrogen desorption.
・Hydrogen absorption of MH is exothermic process, and hydrogen desorption of MH is endothermic process.
Advantages of Metal Hydride
・MH can absorb hydrogen at moderate temperature (below 100 degree C).
・MH can absorb hydrogen at relatively low pressure (below 10 atm).
・MH do not easily explode.
・Space saving: about 1/1000 volume of atmospheric pressure gas.
Disadvantages of Metal Hydride
・Heavy
Comparison of hydrogen storage
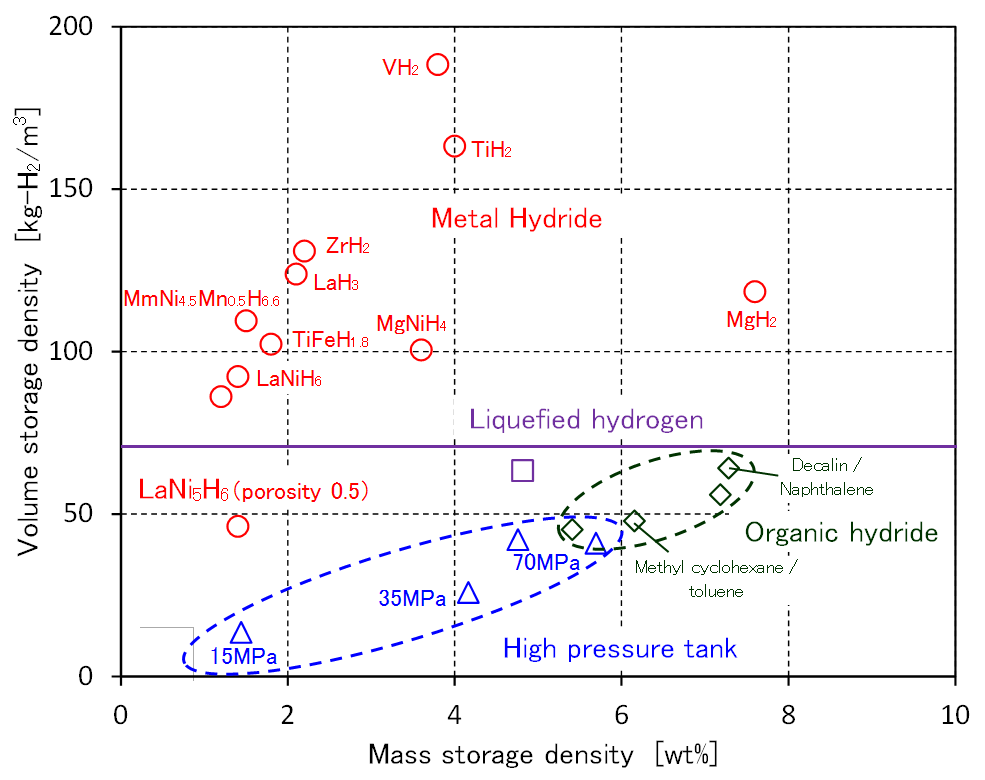
LaNi5
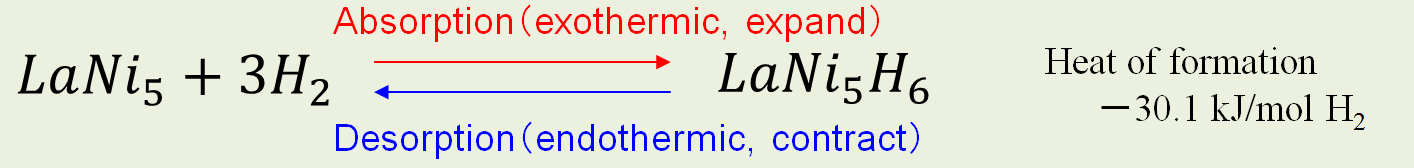
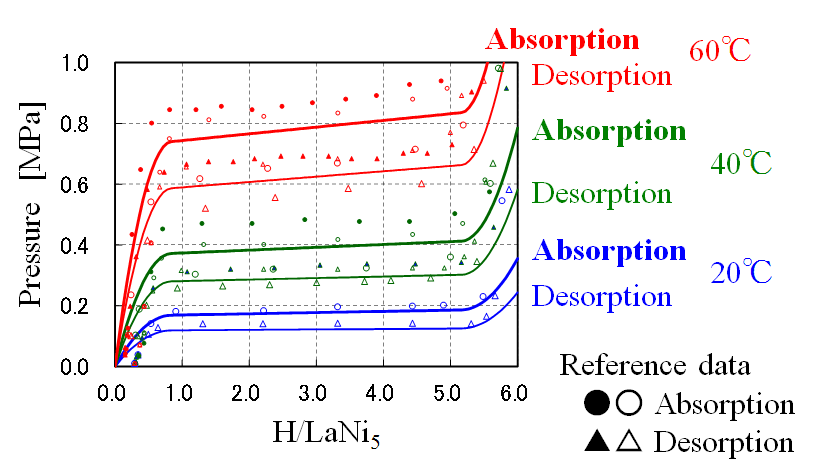
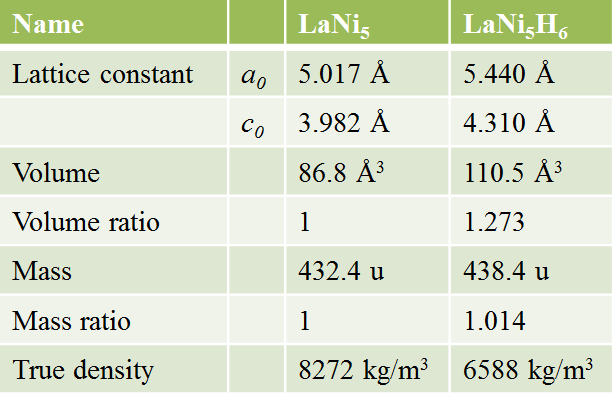
Expansion and contraction due to hydrogen absorption and desorption